Unraveling
the Mysteries of Naming Enantiomers: The R/S System of Stereochemistry
Have you ever wondered how chemists distinguish between the mirror-image forms of chiral molecules? If so, you've stumbled upon the captivating world of stereochemistry. In this fascinating branch of organic chemistry, we find ourselves exploring the spatial arrangements of atoms in molecules, particularly when they possess chiral centers.
To precisely name these enantiomers and understand their unique configurations, chemists employ a powerful nomenclature system known as the R and S notation. Let's embark on a journey of discovery to uncover the magic behind this system!
The
Need for Nomenclature
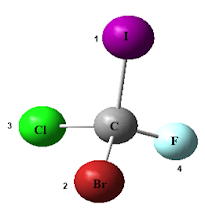
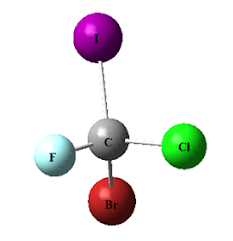
Imagine a compound like 2-bromobutane, a
seemingly simple molecule that exists in two distinct mirror-image forms,
called enantiomers. To communicate the configuration of the asymmetric carbon
in 2-bromobutane accurately, we require a systematic nomenclature. Fortunately, chemists
utilize the letters R and S to signify the configurations about asymmetric
carbons. If a molecule possesses one asymmetric carbon, a pair of enantiomers
will arise—one with an R configuration and the other with an S configuration.
This ingenious nomenclature system, known as the R,S system, was devised by the
brilliant minds of Cahn,
Ingold, and Prelog, revolutionizing the way we understand chirality.
Determining
Configuration: A Three-Dimensional Adventure
To name individual stereoisomers using
the R and S notation,
1. We
must first rank the groups or atoms bonded to the asymmetric carbon in order of priority. The higher
the atomic number of the atoms directly attached to the asymmetric carbon, the
higher their priority. This prioritization concept might ring a bell, as it's
akin to the principles
used in the E, Z system of nomenclature—a system inspired by the R,S
system.
2. Next,
we orient the molecule so that the group or atom with the lowest priority (assigned
number 4) is directed away from us. Then, we draw an imaginary arrow from the
group or atom with the highest priority (assigned number 1) to the group or
atom with the next highest priority (assigned number 2). Here's the captivating
part: If the arrow points clockwise, the asymmetric carbon has the R
configuration (where R stands
for "rectus," Latin for "right"). Conversely, if the arrow points
counterclockwise, the asymmetric carbon has the S configuration (where S stands for
"sinister," Latin for "left")
Visualizing
Spatial Relationships: An Imaginative Adventure
For those who can easily visualize
spatial relationships, the above rules are sufficient to determine whether an
asymmetric carbon has the R or S configuration. Simply rotate the molecule
mentally, making sure the lowest-priority group faces away from you, and draw
the arrow as described.
For those who need a bit more help,
we've got you covered! Perspective
formulas can be a bit tricky, but with the right approach, we can name
enantiomers without the need for mental rotations. Let's demonstrate this with
a thrilling example involving 2-bromobutane.
1. First,
rank the groups bonded to the asymmetric carbon in order of priority. In
2-bromobutane's enantiomers, bromine has the highest priority (assigned number
1), the ethyl group comes second (assigned number 2), the methyl group follows
(assigned number 3), and hydrogen has the lowest priority (assigned number 4).
2. Now,
if the group with the lowest priority (assigned number 4) is bonded by a
hatched wedge, draw an arrow from the group with the highest priority (assigned
number 1) to the group with the second highest priority (assigned number 2). If
the arrow points
clockwise, the compound has the R configuration. If it points counterclockwise, the
compound has the S configuration.
3. However,
if the group with the lowest priority (assigned number 4) is not bonded by a
hatched wedge, a thrilling twist awaits! Switch two groups, so group 4 is now
bonded by a hatched wedge, and then proceed as described above. By doing this,
we determine the configuration of the enantiomer of the original molecule. If the arrow points clockwise,
the enantiomer (with the switched groups) has the R configuration, meaning the
original molecule has the S configuration. On the other hand, if the arrow points
counterclockwise, the enantiomer (with the switched groups) has the S
configuration, meaning the original molecule has the R configuration.
4. As
you master this unique system, remember one essential rule: In drawing the
arrow from group 1 to group 2, you can draw past the group with the lowest priority (assigned
number 4) but never past the group with the next lowest priority (assigned
number 3).
By embracing the R and S notation,
chemists embark on a captivating journey into the world of chirality. With an
understanding of molecular configurations, we can unlock the secrets hidden in
seemingly simple compounds and reveal their enigmatic mirror-image forms. The
R,S system empowers chemists to navigate the three-dimensional labyrinth of
organic molecules and opens up new vistas of exploration in the realms of
pharmaceuticals, materials science, and beyond.









No comments:
Post a Comment