Resonance
Concept:
The
process in which different
Lewis structures can be written for a compound which involve identical positions of atoms
is called resonance. The actual
structure of a compound is considered to be a weighted average of all the contributing
structures. The representation of real structures as a weighted average
of two or more contributing structures is called resonance. These structures are also called resonance contributing structures or canonical forms. The actual
structure is a resonance
hybrid of all these structures. The resonance hybrid resembles each of
the contributing structures but is identical to none of them.
Representation
A double-headed arrow (↔) is placed between each pair of contributing structures. For example, there are various contributing structures of benzene:
(Resonance
Structures of Benzene with percentages shown for Kekulé structures and Dewar
structures)
Obviously,
the two Kekulé structures
contribute largely to the resonance hybrid. Hence, benzene is a
resonance hybrid, principally, of two Kekulé structures.
Resonance
Energy (Delocalization Energy):
The
difference in energy between the resonance hybrid and the contributing structures of the lowest energy is
called resonance (delocalization) energy.
The Rules of Resonance
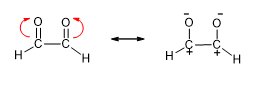
1. Resonance structures are not actual structures of the molecule. They are non-existent, hypothetical, and exist only on paper. They are useful to describe many radicals and ions for which a single Lewis structure is inadequate.
2. Maximum no. of bonds formed by atoms are:
C=4, N=4, O=3, H=1, X=2
(Example structures with resonance including Acetate ion and Skeletal forms)
Note that when nitrogen bears a unit positive charge, it has four covalent bonds.
3. In writing resonance structures, we are only allowed to move electrons, the atomic nuclei must have the same positions in all canonical forms.
(Examples
including Acetoacetic ester and other structures)
4. All
canonical forms must have
the same number of unpaired electrons.
(Examples
of conjugated systems shown)
5. The actual molecule,
i.e., the resonance
hybrid, is always more stable than any of its canonical forms. This is
because, in any one canonical form, each pair of bonding electrons is localized only between two
atoms, whereas in the resonance
hybrid the π-electrons are delocalized over three or more atoms. The
delocalization of π-electrons accounts for the extra stability of the hybrid.
(Example:
Resonance forms of allyl group)
6. All atoms involved in in resonance must lie in a
plane. The reason for planarity is to allow maximum overlap of te p-orbitals
for the delocalization of electrons.
Further
Explanation of Resonance Stability:
7. Not all resonance structures contribute equally to the actual molecule. Each structure contributes in proportion to its stability. The more stable the resonance structure, the more it contributes. The following rules may be helpful in making decisions about the relative stabilities of resonance structures.
a. Structures with more covalent bonds are generally more stable than those with fewer.
b. Structures in which a charge is separated are less stable than those without
charge separation.
d. A structure with two like charges on adjacent carbons is
highly unfavorable.
e. Structures with a negative charge on a more electronegative atom are more stable
than those with the charge on a less electronegative atom.
f. Equivalent resonance structures contribute equally to the resonance hybrid.
g. Greater the number of principal contributing structure of a molecule, the greater the stability.
For example, Nitrate ion is more stable than nitrite ion. Acetate ion is more
stable than acetic acid.














No comments:
Post a Comment