Nomenclature of
Alkanes (IUPAC Rules):
The
IUPAC (International Union of Pure and Applied Chemistry)
system provides systematic rules for naming alkanes and other organic
compounds. Here are the basic rules for naming alkanes:
Rule 1: Identify the longest continuous carbon chain in the molecule. This chain is referred to as the "parent chain," and its length determines the base name of the molecule (e.g., methane, ethane, propane, etc.).
Rule 2:
Number the carbon atoms in the parent chain starting from the end that gives
the lowest possible number to the substituents (groups
attached to the main chain). This ensures that the substituent's position is as
low as possible in the name.
Rule 3:
If the molecule has identical substituents, they
are listed only once in the name, but a prefix is used to indicate how many of
them are present (e.g., di-, tri-, tetra-). For
example, "2,2-dimethylbutane" means there are two methyl groups
attached to carbon atom 2 in the butane chain.
Rule 4:
When the molecule has additional branches (side
chains), these branches are named as alkyl groups, and their positions are
indicated by numbers corresponding to their attachment point on the parent
chain. Complex alkyl groups are enclosed in parentheses
to avoid confusion.
Rule 5:
When numbering the chain produces two possible sets of
numbers, the set that gives the lowest
individual number at the first point of difference is chosen.
·
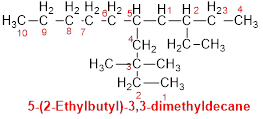
Example:
For the molecule 4,4,5-triethyl-3,5-dimethyloctane:
o The
parent chain is "octane" (8 carbons).
o There
are three ethyl groups attached at positions 4, 4, and 5.
o There
are two methyl groups attached at positions 3 and 5.





No comments:
Post a Comment