"Navigating
the Complexity of Regioselective and Stereoselective Cycloadditions: Challenges
and Opportunities"
Cycloaddition reactions are an important
class of reactions in organic chemistry that involve the formation of cyclic
compounds by the joining of two or more molecules. These reactions have found
widespread application in the synthesis of complex
organic molecules, and are widely used in the pharmaceutical,
agrochemical, and materials
industries. In particular, regioselective
and stereoselective cycloaddition reactions are
highly desirable, as they allow for the selective synthesis of specific isomers
of the desired product.
Regioselective
cycloaddition reactions:
Regioselective cycloaddition reactions
involve the selective formation of a bond
between two specific atoms within the reactant molecules. This can be achieved
by using specific reagents or by controlling reaction
conditions such as temperature, pressure, and solvent.
Stereoselective
cycloaddition reactions:
Stereoselective cycloaddition reactions involve
the selective formation of a specific stereoisomer of
the product. This can be achieved by controlling the
orientation of the reactant molecules with respect to each other, or by
using chiral reagents.
Examples:
Regioselective and stereoselective
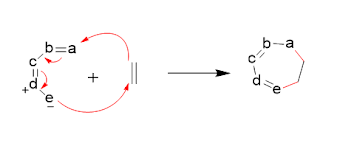
cycloaddition reactions can involve different types of reactions, including [4+2] and [3+2] cycloadditions.
In a [4+2] cycloaddition, a diene and a dienophile react to form a six-membered
ring, while in a [3+2] cycloaddition, a dipolarophile and a dipole react to
form a five-membered ring.
One important class of regioselective
and stereoselective cycloaddition reactions is the Diels-Alder reaction, which
is a [4+2] cycloaddition between
a diene and a dienophile. This reaction is widely used in organic synthesis,
and can be highly selective under the right conditions. For example, by
controlling the electronics and steric hindrance of the reactants, as well as
the reaction conditions, it is possible to selectively form one regioisomer
and/or stereoisomer of the product.
Another important class of
regioselective and stereoselective cycloaddition reactions is the 1,3-dipolar cycloaddition, which is a [3+2] cycloaddition between a dipole
and a dipolarophile. This reaction is used to synthesize a wide range of
heterocyclic compounds, and can also be highly selective under the right
conditions. For example, by using chiral dipoles or
dipolarophiles, it is possible to selectively form specific
stereoisomers of the product.
Strategies:
There are several strategies that can be used to achieve regioselective and stereoselective cycloaddition reactions.
- One approach is to use asymmetric catalysts, which can promote the formation of specific stereoisomers of the product. These catalysts can be chiral ligands or enzymes, and are often highly selective, allowing for the synthesis of complex chiral molecules.
- Another strategy is to use substrates that are pre-functionalized in a way that allows for selective cycloaddition. For example, dienes can be protected with specific groups that prevent the formation of undesired products, or can be selectively activated to promote the formation of specific stereoisomers of the product.
Challenges:
Achieving regioselective and
stereoselective cycloaddition reactions can be challenging, as there are often
competing reactions that can lead to the formation of undesired products. One
challenge in achieving regioselective and stereoselective cycloaddition reactions
is that the reaction mechanism can be complex,
involving multiple transition states and intermediates. As a result, it can be difficult to predict the selectivity of a given reaction, and experimental optimization is often required. However, computational
methods can be used to predict the
regio- and stereochemistry of a reaction, and can be a useful tool in the
design of selective cycloaddition reactions.
Which
computational methods can be used to predict the regio- and stereochemistry of
a reaction?
There are several computational methods that can be used to predict the
regio- and stereochemistry of a reaction, including density
functional theory (DFT), molecular mechanics (MM), and quantum mechanics/molecular
mechanics (QM/MM) methods.
DFT is a widely used method that can
provide accurate predictions of the electronic and
geometric properties of molecules and reactions. In DFT calculations,
the electronic structure of a molecule is described by solving the Schrödinger equation for the electron density, and the
resulting energy is used to predict the structure and energetics of the reaction.
MM methods, on the other hand, use
classical mechanics to describe the motion of atoms in
a molecule, and can provide a fast and efficient way to explore the conformational space of a molecule or a reaction. MM
methods can be combined with DFT or other quantum mechanical methods to model
the electronic properties of a reaction, as well as to include solvent effects.
QM/MM methods combine both quantum
mechanical and classical mechanical calculations to model the electronic properties of a small part of the molecule
(usually the reactive site) with high accuracy, while using classical mechanics
to describe the rest of the molecule. QM/MM methods are particularly useful for
modeling reactions in complex environments, such as enzymes or solvated
systems.
In addition to these methods, there are
also several software packages and databases that can be used to predict the
regio- and stereochemistry of a reaction. These include programs such as Gaussian, ORCA, and MOPAC, as well as databases
such as Reaxys and Scifinder.
Conclusion:
In conclusion, regioselective and
stereoselective cycloaddition reactions are important tools for the synthesis
of complex organic molecules, and are widely used in the pharmaceutical, agrochemical,
and materials industries. However, achieving selective reactions can be
challenging, and requires careful control of reaction conditions, as well as
the use of specialized reagents and catalysts. Despite these challenges, the
development of new strategies for achieving regioselective and stereoselective
cycloaddition reactions continues to be an active area of research in organic
chemistry.