"Exploring
the Fascinating World of Cycloadditions: Unlocking Limitless Possibilities in
Organic Synthesis"
Introduction:
Cycloadditions
are an important class of chemical reactions in which “two or more unsaturated molecules combine to form a cyclic product”. There is a net
reduction of the bond multiplicity.
The
rearrangement of the π-electrons occur and
forming two new σ-bonds. These
reactions are of great importance in synthetic organic
chemistry and are widely used in the preparation of natural products,
pharmaceuticals, and other complex molecules. In this article, we will provide
a detailed overview of cycloadditions, including their mechanism, types, and
applications.
Mechanism
of Cycloadditions:
Cycloadditions typically proceed through
a concerted
mechanism, which involves the formation of a cyclic transition state that is stabilized by the interaction of the reacting molecules' orbitals. This
mechanism is characterized by the simultaneous formation of two or more new bonds and the breaking
of two or more existing bonds. The concerted mechanism of cycloadditions is a
key factor in their high stereoselectivity, as it allows for the
formation of only one stereoisomer of the
product.
Types
of Cycloadditions:
There are several types of cycloadditions;
- [1,3] dipolar cycloadditions
- [2+2] cycloadditions
- [4+2] cycloadditions
- [6+4] cycloadditions
- [3+2] cycloadditions
- [5+2] cycloadditions
- [8+2] cycloadditions
1,3-Dipolar cycloaddition is a type of
chemical reaction in which a 1,3-dipole, such as a nitrene, carbene, or
diazoalkane, reacts with a dipolarophile, such as an alkene or alkyne, to form
a five-membered heterocycle. The reaction proceeds through a concerted
mechanism, in which the 1,3-dipole and the dipolarophile react simultaneously
to form the product.
The [4+2] cycloaddition,
also known as the Diels-Alder reaction, is perhaps the most well-known and
widely used cycloaddition. Diels-Alder reaction is highly stereospecific
reaction. This reaction involves the reaction of a diene, a molecule with two double bonds, with a dienophile, a molecule with a double
bond, to form a six-membered ring. Here, are few examples;
[2+2]
cycloaddition
The [2+2] cycloaddition involves the
reaction of two unsaturated molecules, typically
alkenes, to form a four-membered ring. This
reaction is of great importance in the synthesis of complex
natural products, such as steroids and terpenes.
[3+2]
cycloaddition:
The [3+2] cycloaddition, also known as
the azide-alkyne
cycloaddition, involves the reaction of an azide and an alkyne to
form a triazole ring. This reaction is widely
used in the preparation of pharmaceuticals and
other biologically active molecules.
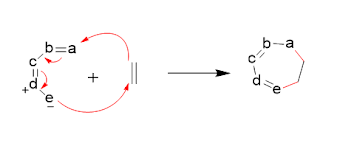
[6+4] cycloaddition:
Ths involves the reaction of
heptatrienone and an cyclopentadiene to form a product. The
exo product is typically favored over the endo product in a [6+4] cycloaddition reaction
because the formation of the exo product is generally more thermodynamically
favorable due to the release of ring strain in the 6-membered ring.
Additionally, the exo product is usually the kinetic product, meaning that it
is formed faster than the endo product due to steric effects and other factors.
Periselectivity
refers to the preference of the reaction to occur at a specific position on the
reactant molecules, resulting in the formation of a specific product.
The frontier molecular
orbital approach for this cycloaddition is given below;
[5+2]
cycloaddition:
The [5+2] cycloaddition is a less common type of cycloaddition that involves the
reaction of a pentadiene and a dienophile to form a seven-membered ring. This
reaction is of great importance in the synthesis of natural products, such as
the polycyclic ether polyketide macrolides.
[8+2]
cycloaddition:
An [8+2] cycloaddition is a type of
chemical reaction in which ring is formed from two separate reactant molecules.
The reaction involves the combination of a diene,
which has two double bonds, and a two-carbon dienophile,
that can react with the diene. The reaction proceeds through a concerted mechanism.
Applications
of Cycloadditions:
Cycloadditions are of great importance
in synthetic organic chemistry and are widely used in the preparation of complex molecules. They are particularly useful in the
synthesis of natural products and pharmaceuticals, as they allow for the
construction of complex ring systems with high stereoselectivity.
One of the most important applications
of cycloadditions is in the synthesis of steroids, which are a class of
biologically active molecules that play a vital role in the regulation of
various physiological processes. The [2+2] cycloaddition is particularly
important in the synthesis of steroids, as it allows for the construction of
the four-membered ring system that is characteristic of many steroid molecules.
Cycloadditions are also widely used in
the synthesis of other biologically active molecules, such as alkaloids and
polyketide macrolides. The [4+2] cycloaddition, in particular, is of great
importance in the synthesis of polyketide macrolides, which are a class of
natural products with potent antibiotic and antifungal activity.
Conclusion:
Cycloadditions are an important class of
chemical reactions that are widely used in synthetic organic chemistry. These
reactions allow for the construction of complex ring systems with high
stereoselectivity, making them particularly useful in the synthesis of natural
products and pharmaceuticals.









No comments:
Post a Comment