Topic: "Introduction of Pericyclic Reactions"
Pericyclic reactions are a class of organic
reactions in which the bond-forming and bond-breaking
events occur in a concerted manner through a cyclic transition state. The
important feature is neither ions nor radicals are formed as intermediates. These
reactions have been studied extensively in organic chemistry and play an
important role in the synthesis of complex organic molecules.
An example is the
reaction between butadiene and propenal which occurs on heating.
One of the most
important classes of pericyclic reactions involves the conjugated polyene
system. A conjugated polyene is a molecule that contains alternating double and
single bonds in a linear arrangement. The delocalized π-electrons in the conjugated
system give rise to unique chemical properties that can be exploited in
pericyclic reactions.
Transition
state:
A state corresponding
to the highest potential energy along this reaction coordinate. It is often
marked with the double dagger ‡ symbol.
Background:
Several pericyclic
reactions, including the Diels-Alder reaction (1928),
the Cope rearrangement (1940), and the Claisen rearrangement (1912), were independently
known.
The key characteristics
of ionic and radical reactions were fully understood by the end of the 1950s.
Pericyclic reactions, however, were not acknowledged as a distinct class.
Doering referred to
them as "No-mechanic reaction" in the
1960s.
Several reactions'
riddles remained unsolved.
- ü Comparing
the ratio of ethylene molecules to cyclobutane with that of acetylenes/arenes
to four-membered rings
- ü changing cis, trans-butadiene from cis-3,4-dimethylcyclobutene
- ü pentadienyl
cation cyclization
R. B. Woodward came
across disrotatory electrocylization, commonly known as "counter thermodynamic stereochemistry," in 1963.
A set of guidelines
defining the stereochemistry of distinct groups of pericyclic reactions were
first introduced in 1965 by R. B. Woodward and R.
Hoffmann.
Based on the symmetry
of the molecular orbitals, Abraham and Longuet-Higgins provided
an explanation of the correlation diagrams.
For this specific group
of reactions, the term "pericyclic" was first used in 1969, setting
the guidelines.
Characteristics:
- Cyclic transition phase
- There is little to no solvent impact.
- Certainly stereospecific
- Neither electrophiles nor nucleophiles are involved.
- No cationic, anionic, or radical intermediates are used.
- Photochemical promotion
is used more frequently while reaction can also be heat catalyzed.
Classification:
Generally,
classified into following classes
Ø Cycloaddition
Ø Electrocyclization
Ø Sigmatropic rearrangements
Ø Group transfer reactions
Cycloadditions:
Concerted cycloaddition reactions are one type of
pericyclic reaction that is commonly used in organic synthesis. In a
cycloaddition reaction, “two or more unsaturated
molecules react to form a cyclic product in which there is a net reduction of the bond
multiplicity”. This is a cyclization reaction.
This is designated as [A+B] where A and B refers to number of atoms containing
π-electrons.
Three important classes of cycloaddition reactions
ü [2+2] Cycloaddition
ü [1,3]-Dipolar cycloaddition
Diels Alder reaction:
In this reaction, conjugated diene and a dienophile
react to form a cyclic product with a six-membered ring. The reaction occurs in a concerted manner,
with the new bonds forming simultaneously.
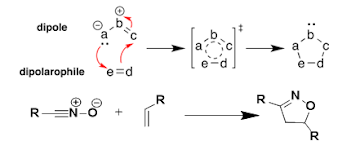
1,3-Dipolar
cycloaddition:
[2+2] Cycloaddition:
Electrocyclic reactions:
Another type of
pericyclic reaction is the electrocyclic addition reaction. In an electrocyclic
addition, a cyclic molecule undergoes a bond-breaking
and bond-forming event to form a new cyclic product. The reaction is initiated
by the absorption of light or heat. The reaction can be either thermally or photochemically driven, and the
stereochemistry of the product is dependent on the conformation of the starting
material.
“Reversible reaction involving
ring closure of a conjugated polyene to a cycloalkene, or ring opening of cycloalkene to a conjugated polyene.”
Example of ring closure
of 1,3,5-hexatriene forms 1,3-cyclohexadiene, a product with one more bond and
one fewer bond than the reactant.
Example of ring
opening of cyclobutene forms 1,3-butadiene, a product with one
fewer bond and one more " bond than the reactant.
Sigmatropic rearrangements:
In a sigmatropic
addition reaction, a group is transferred from one part of the molecule to
another. The reaction occurs in a concerted manner, and the stereochemistry of
the product is dependent on the conformation of the starting material.
“Molecular
rearrangements in which σ-bonded atom or group,
flanked by one or more π-electron systems, shifts to a new location with a corresponding reorganization of the π-bonds are called
sigmatropic reactions.” The total number of σ-bonds and
π-bonds remain unchanged.
§ (3,3)-sigmatropic
rearrangement
§ (2,3)-sigmatropic
rearrangement
§ (1,5)-sigmatropic
rearrangement
Claisen
Rearrangement or 3,3-Sigmatropic rearrangement:
ü This
is example of (3,3) sigmatropic rearrangement having one step mechanism without
ionic intermediate or any charge.
ü Similar
like a cycloaddition reaction.
The π-system in the
conjugated polyene is crucial in these reactions. The π-system allows for the
delocalization of electrons, which in turn leads to increased stability of the
reaction intermediates. The π-system also provides a pathway for the electrons
to move through the molecule, facilitating the formation of new bonds and the
breaking of old ones.
Group transfer reactions:
These reactions are characterized by the transfer of a group from one molecule or from one part of the molecule to
another.
Example of group tarnsfer reaction is ene or alder-ene reaction.
Conclusion:
In conclusion, pericyclic reactions are an
important class of organic reactions that involve the concerted formation and
breaking of bonds through a cyclic transition state. The conjugated polyene
system is an important component of pericyclic reactions, allowing for the
delocalization of electrons and facilitating the formation of new bonds. The
three types of pericyclic reactions discussed above - concerted cycloadditions,
electrocyclic additions, and sigmatropic additions and group transfer - all
rely on the unique properties of the π-system in the conjugated polyene. Understanding
the mechanisms and applications of pericyclic reactions is crucial in the
synthesis of complex organic molecules.













No comments:
Post a Comment