Understanding
Pericyclic Reactions in terms of Symmetry-allowed and Symmetry-forbidden and
Molecular orbital theory:
Pericyclic reactions are a class of
organic reactions that involve a cyclic rearrangement of electrons within a
conjugated system of atoms. These reactions are highly stereospecific and are
governed by the principles of orbital symmetry and molecular orbital theory.
The following key
points are raised in MOT;
§ Molecular orbital can
be described by the linear combination of atomic orbitals (LCAO). In a π molecular
orbital, each electron that previously occupied a p atomic orbital
surrounding an
individual carbon nucleus now surrounds the entire part of the molecule that is
included in the interacting p orbitals.
§ A
p orbital has opposing phases for its two lobes. A covalent bond is created
when two in-phase atomic orbitals come into contact. A node forms between two nuclei when
two out-of-phase atomic orbitals come into contact with one another.
§ The
same principles that govern how electrons fill atomic orbitals—the
aufbau principle, the Pauli exclusion principle, and Hund's rule—apply to how
they fill molecular orbitals: Only two electrons can occupy a given molecular
orbital, and an electron enters the accessible molecular orbital with the
lowest energy.
Figure displays an explanation of ethene's molecular orbitals. (One phase of the two lobes of a p orbital is represented by a blue lobe, while the other phase is represented by a pink lobe). Ethene has one π bond, which results in two p atomic orbitals and two π molecular orbitals. A bonding π molecular orbital is produced by the in phase interaction of the two p atomic orbitals and is denoted by Ψ1 (Ψ is the Greek letter psi). The isolated p atomic orbitals have more energy than the bonded molecular orbital. Ethene's two p atomic orbitals are capable of out-of-phase interactions as well. An antibonding π* molecular orbital Ψ2, which has a higher energy than the p atomic orbitals, is produced by the interaction of out-of-phase orbitals. The atomic orbitals interact additively to produce the bonding molecule orbital, whereas they interact subtractively to produce the antibonding molecular orbital. In other words, the interaction between in-phase and out-of-phase orbitals pulls atoms away whereas the interaction between in-phase and in-phase orbitals binds atoms together. The two electrons in ethene are located in the bonding molecular orbital because two electrons can occupy a molecular orbital because electrons live in the available molecular orbitals with the lowest energy. All molecules with a single carbon-carbon double bond are represented by this molecular orbital diagram.
Due to its two conjugated bonds,
1,3-butadiene contains four p atomic orbitals. There are four possible linear
combinations for four atomic orbitals. There are thus four molecular orbitals: Ψ1, Ψ2, Ψ3
and Ψ4 and Oscillations
are retained, as you can see: Four molecular orbitals are created when four
atomic orbitals are combined. The other half are antibonding molecular orbitals
(Ψ3 and Ψ4), and half are bonding molecular
orbitals (Ψ1 and Ψ2). Two electrons are in Ψ1 and two electrons are in Ψ2 because the four electrons
will live in the available molecular orbitals with the lowest energy. Keep in
mind that despite having varying energies, all molecular orbitals are
legitimate and can coexist. All compounds with two conjugated carbon-carbon
double bonds are represented by this molecular orbital image.
For instance, Ψ1 has
three bonding interactions and zero nodes
between the nuclei, Ψ2
has
two bonding interactions and one node
between the
nuclei, Ψ3 has one bonding
interaction and two nodes between the nuclei, and has zero bonding interactions
and three nodes between the nuclei. In 1,3-butadiene,
the highest occupied molecular orbital
(HOMO) is Ψ2 and the lowest
unoccupied molecular orbital (LUMO) is Ψ3. Light of the right wavelength will boost an electron
from a molecule's ground-state HOMO to its LUMO if the molecule absorbs the
light from Ψ2 to Ψ3. At that point, the
molecule is stimulated. The HOMO is Ψ3
and the LUMO is Ψ4 in
the excited state. In a photochemical reaction, the reactant is in an excited
state as opposed to the ground state in a thermal reaction.
You
should be aware that a molecular orbital is bonding if there are more bonding
interactions than there are nodes between the nuclei, and it is antibonding if
there are less bonding contacts than there are nodes between the nuclei.
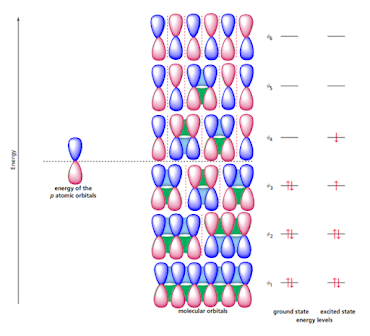
A molecular orbital description of 1,3,5-hexatriene is shown in figure given below;
The concept of symmetry is central to
the understanding of pericyclic reactions, as it can determine whether a
reaction is allowed or forbidden.
Symmetry-allowed
reactions
In terms of molecular orbital, if two p-orbitals
are in phase or both have same lobes they have symmetry allowed. Symmetry-allowed reactions involve
a change in the symmetry of the molecular orbitals in the reactants as they undergo
a cyclic rearrangement of electrons. This change in symmetry must be consistent
with the symmetry of the cyclic transition state. When the symmetry of the
molecular orbitals and the transition state are the same, the reaction is
allowed.
For example, the Diels-Alder reaction
involves the reaction of a diene and a dienophile to form a cyclic product. The
reaction is allowed when the HOMO of the diene and the LUMO of the dienophile
have the same symmetry as the cyclic transition state. This allows for a smooth
flow of electrons through the system, leading to the formation of the cyclic
product.
Symmetry-forbidden
reactions:
In terms of molecular orbital, if two p-orbitals
are out of phase or both have different lobes generating a nodal plane then,
they have symmetry
forbidden. Symmetry-forbidden reactions involve a change in the
symmetry of the molecular orbitals that is not consistent with the symmetry of the
cyclic transition state. These reactions are generally disallowed and do not
occur under normal conditions.
For example, the electrocyclic ring
closure of 1,3,5-hexatriene involves a change in the symmetry of the molecular
orbitals that is not consistent with the symmetry of the transition state. This
results in a symmetry-forbidden reaction that does not occur under normal
conditions.
Methods for explaining pericyclic
reactions:
There are several methods that can be
used to explain the principles of orbital symmetry and the behavior of
pericyclic reactions. These methods include:
Molecular
orbital theory:
This theory describes the behavior of
electrons in a molecule using a set of mathematical functions called molecular
orbitals. Molecular orbital theory can be used to predict the outcome of
pericyclic reactions by analyzing the symmetry and energy levels of the
molecular orbitals involved.
Frontier
orbital theory:
This theory focuses on the highest
occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital
(LUMO) of the reactants in a pericyclic reaction. Frontier orbital theory can
be used to predict the regio- and stereoselectivity of a reaction by analyzing
the overlap of the HOMO and LUMO orbitals.
Woodward-Hoffmann
rules:
These rules provide a set of guidelines
for predicting the stereochemistry of pericyclic reactions based on the
symmetry of the reactants and the transition state.
Conclusion:
In conclusion, energy
of the molecular orbital increases, the number of bonding interactions
decreases and the number of nodes between
the nuclei increases. Understanding the principles of
symmetry-allowed and symmetry-forbidden reactions is crucial for understanding
the behavior of pericyclic reactions. Molecular orbital theory, frontier
orbital theory, and Woodward-Hoffmann rules are important tools for predicting
the outcome of pericyclic reactions and designing new reactions with specific
stereochemical outcomes. By utilizing these methods, chemists can unlock new
possibilities for organic synthesis and materials science.



No comments:
Post a Comment