"Unraveling
the Mysteries of Allyl Group Reactivity with the help of Pericyclic reaction: Exploring
MO Rules and Torqueselectivity"
In this article, we will explore the
understanding of pericyclic reactions using the allyl group as an example, and
explain the concept of torqueselectivity.
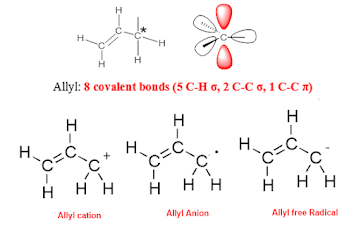
The allyl group is a common functional group in
organic chemistry, consisting of a propene molecule with a single hydrogen atom
replaced by a methyl group. This group is highly
reactive in pericyclic reactions due to its unique electronic structure, which allows for the formation
of π-bonds between adjacent carbon atoms. Allyl groups can participate in a
variety of pericyclic reactions, including cycloadditions, electrocyclic
reactions, and sigmatropic rearrangements.
One of the most well-known pericyclic
reactions involving the allyl group is the Diels-Alder reaction and Claisen rearrangement.
Claisen rearrangement involves the rearrangement of an allyl
vinyl ether to form a new carbon-oxygen bond and a conjugated enone. This
is highly selective for the E-isomer of the
allyl vinyl ether, due to the torqueselectivity
of the reaction.
Half-anti-aromatic systems:
Allylic group contains half-anti-aromatic system. Half-anti-aromatic systems are organic molecules or compounds with a conjugated π-system that contains an odd number of π-electrons, making it partially anti-aromatic in nature.
Anti-aromaticity:
Anti-aromaticity refers to the destabilizing effect that arises from having a
completely conjugated system with 4n π-electrons,
where n is an integer. This destabilization is due to the fact that the delocalized π-electrons in the system do not occupy a
full set of bonding π-orbitals and instead
occupy antibonding π-orbitals, leading to increased energy and decreased
stability.
Half-anti-aromatic systems contain (4n+2) pi-electrons, where n is an integer. The extra
two π-electrons present in half-anti-aromatic systems can occupy a pair of
non-bonding or antibonding pi-orbitals. Because half-anti-aromatic
systems are not completely conjugated, they are less
destabilized than fully anti-aromatic systems.
However, they still exhibit some of the same destabilizing properties, such as
high energy levels and reactivity.
For
conjugated-systems with an odd number of n-carbons, MO diagram rules:
- There are “n” molecular orbitals present in conjugated π-systems with an odd number of n-carbon atoms.
- The system will have (n - 1)/2 bonding, (n - 1)/2 antibonding, and one nonbonding molecular orbital.
- The (n + 1)/2nd orbital, which is always
located between the bonding and antibonding molecular orbitals, is the nonbonding
molecular orbital (ψn).
- The carbon atom(s) of the nonbonding molecular orbitals (n) are traversed by all nodal planes in the system.
- In the case of odd n (i.e., ψ1, ψ3, ψ5 etc.), all nodal planes travel between two carbon nuclei, but in the case of even n (i.e., ψ2, ψ4, ψ6 etc.), one nodal plane passes through the central carbon atom and the remaining nodal planes pass between two carbon atoms.
Allyl Molecular orbital:
Other examples for the conjugated-systems
with an odd number of n-carbons are molecular orbitals of propenyl and pentadienyl
systems. The figure shown below potrayed it.
Allyl-Molecular orbitals-Symmetry:
There are two symmetry elements followed by allylic group;
- Mirror plane (m) symmetry
- C2-axis of symmetry
Torqueselectivity:
In a pericyclic reaction,
torqueselectivity refers to the preference for a
specific stereoisomeric product based on the relative orientation of substituents on the reacting
molecules. This preference arises from the fact that the transition state of
the reaction is highly sensitive to the relative positions and orientations of
the substituents.
- If R is electron donating, then, this filled orbital would unfavorably interact with breaking sigma bond, so, outward opening is favored.
- If R is electron withdrawing, then, this empty orbital would favorably interact with breaking-sigma bond, so, inward opening is favored.
Conclusion:
In conclusion, pericyclic reactions are
important tools in organic synthesis, and the allyl group is a useful
functional group for understanding these reactions. The concept of
torqueselectivity is essential for predicting the outcome of pericyclic
reactions, and can be used to design more efficient synthetic routes.








No comments:
Post a Comment