"Percyclic
Reactions in Hexatriene: Understanding Woodward Rules and Photochemical vs.
Thermal Electrocyclization for Symmetric and Asymmetric Products"
Pericyclic reactions refer to a type of
chemical reaction where a cyclic molecule is formed from a linear molecule
through a series of concerted bond-breaking and bond-forming steps. This type
of reaction is widely studied in organic chemistry, and the molecular orbitals
of the reactants play a significant role in determining the reaction pathway
and product.
One classic example of a pericyclic
reaction is the electrocyclization of hexatriene. This reaction can occur
through either a photochemical or thermal pathway, and the resulting product
can be either symmetric or asymmetric. The reaction mechanism is governed by
Woodward's rules, which describe the allowed and forbidden cyclic transitions
based on the molecular orbitals of the reactants.
The
structure of hexatriene contains 16
covalent bonds (8 C-H σ, 5 C-C σ, 3 C-C π)
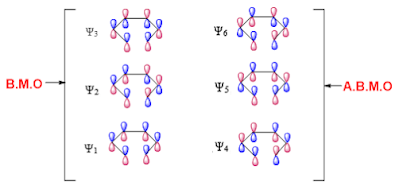
Molecular orbitals for the hexatriene are six named as Ψ1 , Ψ2 , Ψ3 , Ψ4 , Ψ5 , Ψ6 . Among all, first three are bonding molecular orbital (Ψ1, Ψ2, Ψ3) and the remaining are antibonding (Ψ4, Ψ5, Ψ6) in nature.
Notice that, there are two symmetry elements followed by hexatriene;
- Mirror plane (m) symmetry is maintained in thermal conversion of 1,3,5-hexatriene into
1,3-cyclohexadiene
- C2-axis of symmetry is maintained in photochemical conversion of 1,3-cyclohexadiene into 1,3,5-hexatriene or vice versa.
The thermal electrocyclization
reaction of hexatriene is initiated by heating the molecule to a high
temperature. The reaction is also governed by Woodward's rules, but the allowed
and forbidden cyclic transitions are different from the photochemical pathway.
In particular, thermal electrocyclization tends to favor the formation of symmetric products, while
photochemical electrocyclization can result in either symmetric or asymmetric
products. This generates a diradical intermediate, which can undergo a series of concerted bond-breaking and bond-forming steps to form the cyclic product.
In the photochemical electrocyclization of hexatriene, the reaction is initiated by absorption of a photon, which promotes an electron from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). This follows the same mechanism in which a series of concerted bond-breaking and bond-forming steps are involved to form the cyclic product. The resulting product can be either symmetric or asymmetric, depending on the orientation of the substituents on the hexatriene molecule.
We've shown that the mode of
ring closure, which in turn depends on the amount of conjugated bonds present
in the reactant and whether the reaction takes place under thermal or
photochemical circumstances, determines the stereochemistry of an electrocyclic
reaction. Our understanding of electrocyclic processes can be simplified by the
selection
rules stated in
Table. The Woodward-Hoffmann rules for electrocyclic reactions are another name
for these.
For evaluating if a specific electrocyclic reaction is "allowed or forbidden by orbital symmetry," see Table mentioned below.
- If TE (Thermal/Even) describes the reaction, the outcome is given by AC (Antarafacial or Conrotatory).
- If one of the letters of TE is incorrect (it is not Thermal/Even but is Thermal/Odd or Photochemical/Even), the outcome is not given by AC (the outcome is Suprafacial or Disrotatory).
- If both of the letters of TE are incorrect (Photochemical/Odd), the outcome is given by AC (Antarafacial or Conrotatory)—“two negatives make a positive.
In both the photochemical and thermal
pathways, the reaction mechanism is highly dependent on the molecular orbitals
of the reactants. The HOMO and LUMO of hexatriene play a critical role in
determining the allowed and forbidden cyclic transitions, as well as the
orientation of the substituents in the final product. The Woodward rules
provide a useful framework for understanding the reaction mechanism and
predicting the outcome of the reaction.
Conclusion:
In conclusion, the electrocyclization of
hexatriene is a classic example of a percyclic reaction, which can occur
through either a photochemical or thermal pathway. The resulting product can be
either symmetric or asymmetric, depending on the orientation of the
substituents on the hexatriene molecule. Woodward's rules provide a useful
framework for understanding the reaction mechanism and predicting the outcome
of the reaction.








No comments:
Post a Comment