"Extraction
of Nicotine from Tobacco Leaves"
Theory:
Nicotine is an alkaloid primarily found
in tobacco (Solanaceae Family), as well as in smaller quantities in tomato
plants and green peppers. It is also present in the Coca plant. Nicotine was
first isolated from tobacco plants in 1828 by German chemists Posselt and
Reimann. It is a potent neurotoxin and is used in various insecticides.
Apparatus
Required:
- 250 ml beaker
- Separatory funnel
- Measuring cylinder
- Hot plate
- Magnetic stirrer
- Weighing balance
- Iron stand
- Pipette
Chemicals
Required:
- 5% NaOH solution
- Diethyl ether
- Tobacco leaves
- Distilled water
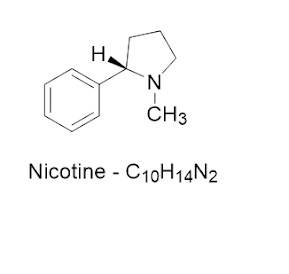
Structure:
Procedure:
- Clean and dry all the apparatus carefully.
- Take 10g of tobacco leaves, wash them thoroughly, and ensure they are completely dried.
- Grind the leaves and add them to a solution of 100g 5% NaOH.
- Stir the mixture thoroughly and filter the solution.
- Dilute the filtrate with 30ml of distilled water to remove impurities.
- Heat the solution on a hot plate until it boils and a strong smell of nicotine is obtained.
- Transfer the solution into a separatory funnel and extract it by adding 25ml of diethyl ether. Repeat the extraction process three times.
- Evaporate the ether using a hot plate placed in an ice bath.
- Separate the upper brown layer of nicotine oil.
Result:
The yield of nicotine oil obtained was 3.8 ml.
Calculations:
Initial amount of tobacco leaves = 10
grams
Concentration of nicotine in the
extracted oil = 5%
Step
1:
Calculate the amount of nicotine in the
extracted oil: Amount of nicotine = Volume of nicotine oil * Concentration of
nicotine Amount of nicotine = 3.8 ml * 5% (0.05) Amount of nicotine = 0.19
grams
Step
2:
Calculate the percentage yield:
Percentage yield = (Amount of nicotine /
Initial amount of tobacco leaves) * 100
Percentage yield = (0.19 g / 10 g) * 100
Percentage yield = 1.9%
Therefore, with the new assumptions, the
percentage yield of nicotine oil would also be approximately 1.9%.
Observations:
Molecular
Formula: C₁₀H₁₄N₂
Molar
Mass: 162.23 g/mol
Color:
Nicotine is a colorless to pale yellow liquid in its pure form.
Melting
Point: Nicotine has a melting point of -79°C (-110°F).
Boiling
Point: Nicotine has a boiling point of 247°C (477°F) at
normal atmospheric pressure.
Precautions:
- Always wear gloves while conducting the experiment.
- Use a mask to protect against the pungent smell of nicotine oil.
- Ensure thorough cleaning and drying of apparatus before use.
- Handle nicotine with caution due to its neurotoxic properties.
- Follow proper disposal protocols for chemical waste generated during the experiment.




No comments:
Post a Comment