A
Comprehensive Guide: Frontier Molecular Orbital Approach and Perturbational
Molecular Orbital Approach for Sigmatropic Rearrangements:
Sigmatropic rearrangements are a class
of organic reactions in which a sigma bond undergoes
a shift, resulting in the formation of a new sigma bond
and the breaking of an old one. These
reactions are ubiquitous in organic chemistry and have been extensively studied
over the years. Two approaches that have been employed to understand the
mechanism of sigmatropic rearrangements are the Frontier
Molecular Orbital (FMO) approach and the Perturbational
Molecular Orbital (PMO) approach. In this article, we will provide a
comprehensive guide on both these approaches and their applications in the
context of sigmatropic rearrangements.
Frontier
Molecular Orbital Approach:
The Frontier Molecular Orbital (FMO)
approach is a theoretical method that utilizes
the concept of frontier molecular orbitals to explain the reactivity of organic molecules. The FMO approach is
based on the Hückel molecular orbital theory,
which describes the electronic structure of π-conjugated systems. According to
this theory, π-conjugated systems have a set of molecular orbitals that are
energetically close to each other, known as the frontier
molecular orbitals. These orbitals are the highest
occupied molecular orbital (HOMO) and the lowest
unoccupied molecular orbital (LUMO).
In the FMO approach, the HOMO-LUMO
energy gap is used to predict the reactivity of a
molecule towards a particular reaction. For sigmatropic rearrangements,
the FMO approach is used to determine the thermal and
photochemical reactivity of the reactant molecules. The approach
provides insights into the preferred direction of the reaction, the activation
energy, and the stereochemical outcome of the rearrangement.
Here are some examples;
Mechanism
Here the noteable thing is 1,5 sigmatropic
rearrangement occuring thermally is symmetrically allowed While 1,5 sigmatropic
rearrangement occuring photochemically gives 1,3 product.
[1,
3] Sigmatropic Rearrangement:
[1, 3] Sigmatropic Rearrangement is
found in allylic compounds. The rearrangement is given below;
Keep in mind; Allylic HOMO is asymmetric and antarafacial in ground state while symmetric and Suprafacial in excited state.
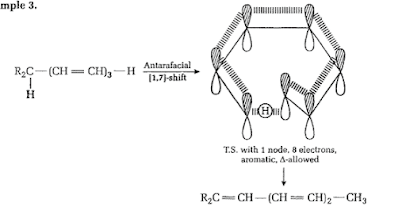
[1, 5] Sigmatropic Rearrangement is found in Pentadienyl compounds. The rearrangement is given below;
Selection
rules for Sigmatropic Shifts:
Perturbational
Molecular Orbital Approach:
The Perturbational
Molecular Orbital (PMO) approach is another theoretical
method used to study sigmatropic rearrangements. The PMO approach is
based on the idea that the reaction proceeds through a transition
state that is formed by the interaction between the reactant and the
product molecules. The interaction between these molecules can be described by perturbing the electronic structure of the reactant
molecule.
In the PMO approach, the reactant
molecule is perturbed by a small amount, and the resulting electronic changes
are used to calculate the interaction energy between
the reactant and the product molecules. This interaction energy is then used to
determine the activation energy and the transition state geometry of the rearrangement. The
PMO approach is particularly useful for understanding the effects of substituents on the reactivity of the reactant molecule.
Here are some examples showing the PMO
approach;
[1, 3] Sigmatropic Rearrangement:
Selection rules for Sigmatropic Shifts:
Applications
of FMO and PMO Approaches in Sigmatropic Rearrangements
The FMO and PMO approaches have been
extensively used to study sigmatropic rearrangements in various systems. One of
the most well-known applications of these approaches is the Cope rearrangement. The Cope rearrangement is a
sigmatropic rearrangement that involves the migration of a vinyl group from one
carbon to another in a cyclohexadiene system. The FMO approach has been used to
predict the preferred direction of the rearrangement, and the PMO approach has
been used to determine the transition state geometry.
Another important application of the FMO
and PMO approaches is in the study of the Diels-Alder reaction. The Diels-Alder reaction is a cycloaddition reaction that
involves the formation of a new sigma bond between a diene and a dienophile.
The FMO approach has been used to predict the regioselectivity and the
stereochemistry of the reaction, while the PMO approach has been used to
determine the effects of substituents on the reactivity of the reactant
molecules.
Conclusion
In conclusion, the Frontier Molecular
Orbital (FMO) approach and the Perturbational Molecular Orbital (PMO) approach
are two powerful theoretical methods used to study the mechanism of sigmatropic
rearrangements. Both approaches provide valuable insights into the reactivity, activation energy,
and transition state geometry of these reactions. The FMO approach is
particularly useful for predicting the thermal and photochemical reactivity of
reactant molecules, while the PMO approach is useful for understanding the effects of substituents on the reactivity of
reactants. These approaches have found wide applications in studying various
sigmatropic rearrangements, including the Cope
rearrangement and the Diels-Alder reaction. Overall, the FMO and PMO
approaches are important tools for understanding the fundamental principles of
organic chemistry and can aid in the design of new reactions and synthetic
strategies.













No comments:
Post a Comment