Preparation
of Schiff’s Base from benzaldehyde
Theory:
Schiff
bases are a class of compounds that contain a carbon-nitrogen
double bond with an imine functional group
(R-N=CR'). They are typically synthesized by condensation reactions
between a primary amine and an aldehyde or ketone. Schiff bases find
applications in coordination chemistry, organic
synthesis, metal ion detection, biological activities, dye chemistry,
optoelectronic devices, corrosion inhibition, and chelation. They are
versatile compounds with uses ranging from catalysis and
drug development to sensing and materials
science. In this case, we
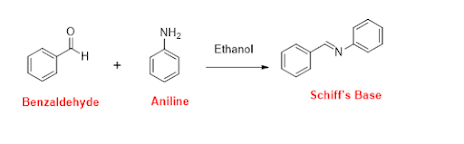
will be synthesizing a Schiff base by reacting aniline (primary amine) with
benzaldehyde (aldehyde) in the presence of ethanol as a solvent.
Chemicals Required:
- Aniline (C6H5NH2)
- Ethanol (C2H5OH)
- Benzaldehyde (C6H5CHO)
Apparatus Required:
- Round-bottom flask
- Magnetic stirrer
- Ice bath
- Heating source (e.g., hot plate)
- Filtration setup (filter funnel, filter paper)
- Weighing balance
- Glassware (beakers, measuring cylinders)
Chemical reaction:
Mechanism:
Procedure:
- Measure 2 mL of aniline (C6H5NH2) using a measuring cylinder and transfer it to a round-bottom flask.
- Add 20 mL of ethanol (C2H5OH) to the round-bottom flask containing aniline. Stir the mixture until the aniline is completely dissolved.
- In a separate container, measure 3.5 mL of benzaldehyde (C6H5CHO).
- Place the round-bottom flask with the aniline-ethanol solution on a magnetic stirrer.
- Add the benzaldehyde dropwise to the aniline-ethanol solution while stirring continuously. The reaction mixture should turn yellow as the Schiff base is formed.
- After the addition is complete, continue stirring the reaction mixture at room temperature for 10 minutes to ensure complete reaction.
- Remove the round-bottom flask from the heating source and allow it to cool to room temperature.
- Set up a filtration setup with a filter funnel and filter paper. Transfer the reaction mixture to a separating funnel. Extract the Schiff base precipitate from it.
- Dry the filtered Schiff base by placing it in an oven at a suitable temperature (e.g., 60°C) or by placing it in a desiccator until a constant weight is achieved.
- Weigh the dried Schiff base to calculate the yield and determine its purity.
Observations:
|
Colour |
pale yellow to dark brown |
|
Crystal shape |
can be diverse, including needle-like
crystals, plate-like crystals, or irregular-shaped crystals |
|
Melting point |
2200C |
|
Boiling Point |
2480C |
Calculations:
To calculate the theoretical yield, we
need to determine the limiting reagent. The reactants are aniline and
benzaldehyde. We will calculate the moles of each reactant and compare them to
find the limiting reagent.
Molar mass of aniline (C6H5NH2)= 93.13
g/mol
Molar mass of benzaldehyde (C6H5CHO):
106.12 g/mol
Moles of aniline = (volume in mL x
density) / molar mass Moles of aniline = (2 mL x 1.021 g/mL) / 93.13 g/mol =
0.0219 mol
Moles of benzaldehyde = (volume in mL x
density) / molar mass
Moles of benzaldehyde = (3.5 mL x 1.044
g/mL) / 106.12 g/mol = 0.0345 mol
Now, let's compare the moles of each
reactant:
Moles of aniline= 0.0219 mol
Moles of benzaldehyde= 0.0345 mol
From the comparison, we can see that aniline is the limiting reagent because it has fewer
moles than benzaldehyde. Therefore, we will use the moles of aniline to
calculate the theoretical yield.
The reaction between aniline and benzaldehyde produces 1 mole of the Schiff base.
Molar mass of C6H5-CH=N-C6H5 = (Molar
mass of C6H5) + (Molar mass of CH) + (Molar mass of N) +
(Molar mass of C6H5)) = (12.01 g/mol x 6) + (1.01 g/mol) +
(14.01 g/mol) + (12.01 g/mol x 6)
Molar mass of Schiff base = 152.19 g/mol
Theoretical yield = Moles of limiting
reagent x molar mass of the Schiff base
Theoretical yield = 0.0219 mol x 152.19
g/mol
Theoretical yield = 3.34 g
Actual yield = 2.8 grams
Theoretical yield = 3.34 grams
Now we can calculate the percentage
yield:
Percentage yield = (Actual yield /
Theoretical yield) x 100
Percentage yield = (2.8 g / 3.34 g) x
100
Percentage yield = 83.83%
Therefore, the calculated percentage
yield of the Schiff base would be approximately 83.83%.


No comments:
Post a Comment