"Exploration of How migration of Hydrogen and migration of Carbon occurs in Sigmatropic Reaction"
Migration
of Hydrogen:
The s orbital of
hydrogen is partially bound to both the migration
origin and the migration endpoint in the
transition state when a hydrogen migrates in a sigmatropic
rearrangement. As a result, a four-membered ring
transition state is involved in a [1, 3] sigmatropic migration of
hydrogen. The HOMO is asymmetric because it
involves two pairs of electrons. Therefore,
according to the selection rules, a [1, 3]-hydrogen shift under thermal conditions requires an antarafacial rearrangement. Because the four-membered ring
transition state does not permit the necessary antarafacial
rearrangement, [1, 3]-hydrogen shifts do not take place under thermal
conditions.
[1,
3]-hydrogen shift:
If the reaction is conducted under photochemical conditions, [1, 3]-Hydrogen shifts may occur because the HOMO is symmetric under these circumstances, allowing hydrogen to migrate via a suprafacial channel.
Due to the fact that two distinct allylic hydrogens can go through a 1,3-hydrogen
shift, the reaction yields two products.
[1,
5]-hydrogen shift:
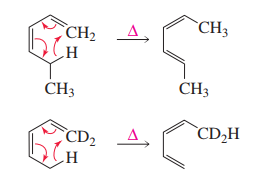
[1,5] Hydrogen sigmatropic migrations
are well recognised. They take place through a
suprafacial pathway under hot conditions because
they involve three pairs of
[1,
7]-hydrogen shift:
In [1,7] sigmatropic hydrogen
migrations, four pairs of electrons are
involved. They are possible because the eight-membered
ring transition state permits the necessary antarafacial
rearrangement in thermal conditions.
Migration
of Carbons:
Carbon has two routes to migrate because
of its two-lobed p orbital, in contrast to
hydrogen, which can only migrate in one direction due to its spherical s orbital. Using one of its lobes, carbon
can communicate with both the migration's origin and
terminus.
If a suprafacial rearrangement is
necessary for the reaction, carbon will migrate utilising either one of its lobes if the HOMO is
symmetric or both of its lobes if the HOMO is
asymmetric.
The migrating group maintains its
configuration when carbon migrates with only one of its p lobes engaging with the migration source and migration terminus
since bonding is always to the same lobe.
Different lobes are involved in bonding in the reactant and in bonding in the
product when carbon migrates with both of its p lobes
engaging.
The following [1, 3]
sigmatropic rearrangement has a four-membered-ring transition state that requires a suprafacial pathway. The reacting system has two pairs of
electrons, so
its HOMO is
asymmetric. Therefore, the
migrating carbon interacts with
the migration
source and the migration terminus using both of its lobes, so it undergoes inversion of configuration.









No comments:
Post a Comment